Coronavirus: What Amyloidosis Patients Should Know
COVID-19 has impacted everyone. Patients with amyloidosis may be at greater risk than the general population. In this frequently updated post, ARC provides the latest recommendations and resources.
Bookmark this page and check back regularly for the latest updates.
April 5, 2022
Should I get a second COVID-19 booster?
On March 29th the FDA and CDC authorized and released updated recommendations, supporting an additional COVID-19 booster dose for individuals over 50 years old or those with a compromised immune system due to an existing medical condition. The Pfizer and Moderna vaccines were both approved for a second booster dose and can be given to people who received their first booster dose at least four months ago.
These newly released recommendations are particularly important for higher risk individuals, such as those with amyloidosis. Throughout the pandemic, data has shown that vaccination and booster doses have offered people protection from infection, hospitalization, and severe COVID-19 outcomes.
As always, it is important to discuss your prevention options with a doctor. Booster doses offer an increase in immunity in the near term, bringing protection levels up and reducing risk for severe illness in the event of a COVID-19 infection. Pairing recommended vaccine and booster guidance with continued mask wearing in situations where there is a heightened risk of exposure to COVID-19, especially in indoor settings, offers the best defense to avoid infection at this time.
With the rise in cases and variants of COVID-19, and the risks posed to some amyloidosis patients, ARC continues to actively monitor recommendations. We will provide the most up-to-date information and resources pertaining to the amyloidosis community on this page. Please check back regularly.
For more information regarding the updated COVID-19 booster recommendations, please see the FDA announcement and visit the CDC website
January 5, 2022
Coronavirus (COVID-19): An Update for Amyloidosis Patients
With the rise in cases and variants of COVID-19, and the risks posed to some amyloidosis patients, ARC continues to monitor recommendations. We will provide the most up-to-date information and resources pertaining to the amyloidosis community in the following blog post. Please bookmark this page and check back regularly for the latest updates.
What do I need to know about variants?
When a person’s body is fighting off a virus, like COVID-19, the virus may try to change or mutate to have the best chance at winning the fight, resulting in a form of the virus that looks and acts differently than the original. These new forms are referred to as variants. Virus variants are common and expected and some may spread more easily or be more resistant to treatments or vaccines. Scientists try to learn as much about new variants as quickly as possible to inform new recommendations. It is important that we continue to follow guidance and implement methods that slow the spread of COVID-19 to keep each other safe. This includes:
- Getting the COVID-19 vaccine and recommended boosters
- Wearing a mask that covers your nose and mouth when in public places
- Staying 6 feet apart from those not in your household
- Avoiding crowds and poorly ventilated indoor spaces
- Getting tested if you have symptoms of COVID-19 or have been in close contact with someone that has tested positive for the virus
- Frequently washing your hands with soap and water or using hand sanitizer if soap and water aren’t available
At this time, it appears as though the most recent variant, referred to as the Omicron variant, is highly contagious, estimated at about 2-4 times more infectious, even among fully vaccinated and boosted individuals. However, individuals who are fully vaccinated and current with the booster appear to experience mild symptoms and are much less likely to be hospitalized for COVID-related symptoms.
Do I need a booster shot?
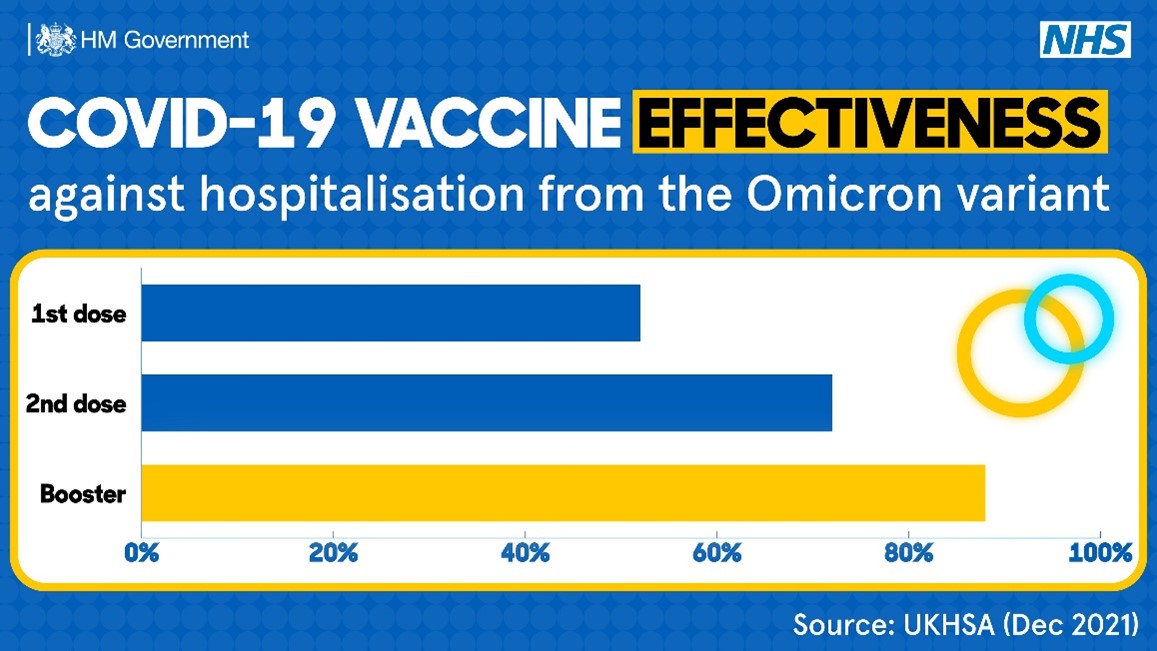
Many people believe that 1 dose of the Johnson & Johnson vaccine, or 2 doses of either Moderna or Pfizer’s vaccines, leave them completely protected. While the vaccines are effective, everyone’s individual immune response is different, especially for those with coexisting medical conditions. Additionally, we know that the effectiveness of the vaccines begins to wane over time. For these reasons, it is recommended that everyone receive a booster. As shown in the graph below, those that receive a booster dose of the vaccine have a higher chance of avoiding hospitalization from the Omicron variant than those with just 1 or 2 doses.
The ARC team asked Dr. Daniel Judge, Professor of Medicine and Director of the Medical University of South Carolina (MUSC) Cardiovascular Genetics Program about booster doses for those with amyloidosis. Dr. Judge said, “COVID19 has been devastating for many people, particularly for people with chronic medical conditions like amyloidosis. Personally, I am glad to have received 2 doses of the vaccine, followed by a booster. I know that this has helped to protect me from serious COVID19 complications. I strongly recommend full vaccination with a booster for all patients with cardiac amyloidosis, with optimal timing to be determined in conjunction with their treating physicians.”
For more information regarding the COVID-19 pandemic, please visit the CDC website and talk to your doctor about the best time to get your COVID-19 vaccination and booster.
June 2, 2021
My state has lifted mask mandates and capacity restrictions – does that mean its safe to return to pre-pandemic life?
A global medical roundtable of some of the world’s leading amyloidosis experts provided insights about patients with transthyretin amyloidosis (ATTR) during the COVID-19 pandemic. The insights, published May 6, 2021, state that patients with ATTR are particularly vulnerable to coronavirus morbidity due to the multisystemic nature of TTR amyloidosis, as well as general older age of the TTR amyloidosis population. The heightened risk of poorer outcomes from COVID-19 in patients with amyloidosis require additional precautions and specialized disease management. Key highlights from the publication are listed here:
- Older patients with ATTR amyloidosis are at increased risk for developing severe COVID-19, requiring social distancing, use of protective masks, and frequent hand washing
- Many older patients with ATTR amyloidosis share comorbidities known to increase morbidity and mortality risk in COVID-19
- Patients with cardiac ATTR amyloidosis should be aware of their predisposition to complications if they develop COVID-19, particularly stroke and cardiac-related issues
- Differential access to care and the utilization of telehealth may more greatly impact older individuals with ATTR amyloidosis
- Laboratory test results, such as elevated cardiac biomarkers, may be seen in both ATTR amyloidosis and COVID-19, confusing interpretation
- Limitations of in-person evaluations and performing diagnostic evaluation during the COVID-19 pandemic limits the ability to diagnose and follow progression of patients with ATTR amyloidosis
- Because of overwhelmed resources as well as safety of patients and healthcare personal, new approaches to clinical research including remote assessments need to be considered
- More research is needed to fill remaining gaps in knowledge to better understand the real-world clinical impact of COVID-19 on patients with ATTR amyloidosis
Given the additional risk of the coronavirus to amyloidosis patients, it is advised that patients continue to take steps to minimize their risk of contracting the virus. To read the full publication from the medical roundtable, click here
As always, keep your health care team informed of any symptoms, treatment and vaccine decisions, and questions you may have about your health.
December 23, 2020
Is the COVID-19 vaccine safe? How effective is it?
The United States Food and Drug Administration (FDA) has granted emergency approval for the COVID-19 vaccine. This essentially means that the FDA granted approval based on less information than is normally required, as the typical process takes years to evaluate a new vaccine. The vaccine is said to be the best hope for ending the pandemic, but given how new it is, the public has had a lot of questions about its safety and efficacy. We have gathered the information that can help keep you informed.
There are a few different companies that are developing a vaccine for COVID-19, and each vaccine will therefore have some differences. The FDA has granted the emergency use authorization to the vaccine developed by Pfizer and BioNTech, which has shown an efficacy of about 95%. Moderna has also developed a vaccine, which has demonstrated 94.1% accuracy. Moderna has applied for FDA emergency use and is expected to receive that authorization soon. AstraZeneca and Janssen are also developing COVID-19 vaccines, but have not yet completed clinical trials.
The vaccines require 2 separate injections given 3 or 4 weeks apart. Neither vaccine uses live COVID-19 viral particles, and therefore cannot give you COVID-19. The COVID-19 vaccines are considered safe. Some people have experienced some mild side-effects from the vaccine including headache, muscle or joint pain, fatigue, and fever.
Should amyloidosis patients receive the COVID-19 vaccine?
ARC has received many inquiries about whether amyloidosis patients should receive the vaccine. We have consulted with many amyloidosis specialists and all have stated that they are advising their patients to receive the vaccine, however they add that each patient should consult with their individual specialist, as other concomitant illness or allergies may be present and the recommendation for that patient may change.
Dr. Angela Dispenzieri, Professor of Medicine and of Laboratory Medicine and Pathology, Mayo Clinic Rochester, shared her thoughts with ARC: “With the first immunizations being given in December 2020, this is hopefully the beginning of the end of the COVID-19 pandemic. We look forward to our patients with amyloidosis receiving vaccinations in the next month or so. We believe it should be safe to them to receive them, although some may not get as protective response as healthier individuals. Despite this historic advance, we will continue to stay safe and smart for several more months to come.”
If I am going to get a COVID-19 vaccine, should I alter or delay my amyloidosis treatment regimen?
At current, specialists do not have reason to believe they will need to change a patient’s treatment regimen or schedule based on when/if they receive a COVID-19 vaccine. However, if a patient has a history of reactions to amyloidosis treatment, a history of reactions to vaccines, or if a patient is just starting a new treatment, it may be wise to wait some time between the vaccine and the treatment.
Should patients who are immunocompromised or who have had an organ transplant get the COVID-19 vaccine?
Earlier this month at the American Society of Hematology’s annual meeting, Dr. Anthony Fauci, the nation’s leading infectious disease expert, stated that patients with compromised immune systems should plan to be vaccinated against COVID-19. In patients with solid organ transplants, the efficacy of the COVID-19 vaccine is unknown. The American Society of Transplantation is recommending that transplant recipients and their household members should receive vaccination when it becomes available. This recommendation is based off previous vaccination guidelines for solid organ transplant recipients. It is thought that patients in both the solid organ transplant population and immunocompromised patient population may not have as robust of an antibody response to the vaccine as the general population. However, as Dr. Fauci also stated at ASH, “some degree of immunity is better than no degree of immunity.”
As always, please check with your healthcare team before making any medical decisions.
ARC remains committed to keeping you informed. Check back here as we continue to collect the latest information on COVID-19, the vaccine, and what this means for amyloidosis patients.
July 30, 2020
ARC’s two webinars in response to the coronavirus pandemic:
Earlier this month, ARC hosted two webinars in response to the COVID-19 pandemic, one for patients and one for physicians. Each webinar included an expert panel to address common questions, special considerations for amyloidosis and high-risk cardiac patients, policy changes that may affect care, and updates to clinical trials. Both webinars were recorded and are now available for viewing!
Patient Webinar: Managing your Amyloidosis during COVID-19
Physician Webinar: Caring for Amyloidosis Patients during COVID-19
Please keep scrolling to view other common questions and recent updates to our coronavirus blog post. We will be updating this continually as new information becomes available.
May 4, 2020
How should I prepare for my telehealth visit?
Watch this short video to learn how to make the most out of your remote visits with your amyloidosis specialists.
Telehealth visits, whether with a video appointment or a simple phone call, have long been a topic of discussion, yet many providers have not adopted this option until now. So, it’s likely many patients don’t yet have experience with having a telehealth visit and therefore don’t know what to expect. After speaking with some amyloidosis experts such as Mat Maurer, MD in New York and Sascha Tuchman, MD in North Carolina who have recently shifted the majority of their appointments to virtual, ARC has been able to gather some great tips for patients on how to prepare for a telehealth visit during the COVID-19 outbreak and what to expect.
Similar to an in-person visit, you might get an appointment reminder a few days before your scheduled appointment or a call from a member of the administrative team to verify your insurance information. Also, it’s possible that a nurse or medical assistant may call you before your appointment to reconcile your medication list and gather other information, but that depends on where you’re seen. If your physician’s office would like to take this approach, they will let you know if you should expect a pre-appointment phone call.
If your physician’s office does not do a pre-appointment phone call, you should still be prepared to provide an update to your medications. You can make the most of your appointment by having the names, dosage, and frequency of all medications ready on a list, or taking photos of them ahead of your appointment.
If you have a scale, thermometer, and/or a blood pressure cuff at home, some physicians may want to collect your weight, temperature, and blood pressure like they would during an in-person visit. If you can safely and accurately gather these values before your appointment you should consider doing so, as this will help your physician track your health.
When you visit your doctor in person, its typical they request you to arrive 15+ minutes before your appointment and you may often experience a wait time if appointments before yours run over. Telehealth appointments can be no different. You should plan to be online and ready for your appointment about 15 minutes before its scheduled time. This will allow time to make sure your internet connection, video, and software are all working properly. You may also need to wait a short time while your physician finishes up a previous appointment.
While the pandemic remains a top concern for most, you should prepare a few main topics that you’d like to discuss with your physician. It can be difficult to remember everything you want to discuss during a regular visit, but now with new visit types to get used to and a global pandemic to worry about, it’s even easier to lose your train of thought or forget to mention something. ARC has developed the Amyloidosis Appointment Companion, a tool to help you organize your thoughts and concerns before an appointment and list your treatment goals to ensure that you are making the most of your time with your physician and addressing all your needs. The Appointment Companion also outlines how you’ve been feeling recently, which is important for your physician to know and understand the whole picture of you, not just how you present on the day of your appointment. The Appointment Companion can also track the list of all your medications to easily share this information with your care team and be prepared for your in-person or telehealth visit. Sign up and create your appointment companion today with My Amyloidosis Pathfinder. Click here to get started.
April 8, 2020
As experts are learning more about the coronavirus, recommendations and guidelines are evolving. The FDA released a comprehensive webpage for patients and caregivers addressing numerous questions and concerns. Highlights from that webpage are provided below, and the full report can be found here.
Should I be wearing a face mask or other face covering?
While it was initially believed that a face mask would not be effective in preventing transmission of the coronavirus, the Centers for Disease Control and Prevention (CDC) are now recommending that face masks be worn to cover the nose and mouth when going out in public. Of course, the safest option, and best practice is to stay home whenever possible. Surgical grade face masks should be reserved for medical personnel, as they are facing a shortage of personal protective equipment (PPE).
The FDA included step-by-step instructions for making your own cloth facemask using a t-shirt or bandana. It is also advised that these cloth masks be washed regularly to ensure that they remain safe and free from germs.
What should I do if I can’t find any hand sanitizer?
The FDA is aware that sanitizing supplies, both for hands and around the house, are hard to come by during these times. Fortunately, the best practice for personal cleanliness and household cleaning is still to use soap and water. Only when soap and water are not available should you rely on hand sanitizer for protection.
Are items produced in a country with high rates of coronavirus at risk for transmitting the virus?
At this time, there is no evidence demonstrating that food or products produced in an affected country can transmit COVID-19.
For more information regarding these topics, potential drug shortages, blood donation, and other COVID-19 information, please visit the FDA webpage here.
April 3, 2020
Can I receive my treatment at home?
Policymakers and insurance companies are recognizing the need for individuals to be able to stay at home as much as possible during this global pandemic, especially those at increased risk. Most physician-administered infusion or injection drugs are considered a specialty medication, and therefore covered under an individual’s medical benefit rather than their prescription benefit. Under the medical benefit, it is common to see the patient being required to go to a hospital or physician’s office to receive the treatment, particularly with Medicare policies. Given the outbreak of COVID-19 and the potential risk of exposure to the virus in medical settings, insurance companies, including Medicare, are relaxing coverage policy guidelines and allowing for patients to receive specialty medication in the comfort of their own home, through a home infusion or home health company on a temporary basis. These policy changes should not be interpreted as a blanket statement that applies to all individuals, just merely a new option to consider that might be available to you. If you feel an infusion at home may be a better option for you than going into an office or medical center, please first consult with your doctor. If your doctor is in agreement with this decision, you should call your insurance company to see if they will allow for temporary home infusion given the current circumstances, or ask your physician’s office for assistance with initiating the process.
March 25, 2020
On Monday, in an interview with the Today Show, the US Surgeon General, Dr. Jerome Adams, warned that “it’s going to get bad” this week in the US. Adams states that people are not adhering to the social distancing recommendations and are in denial that they could get sick from this. “Everyone needs to act as if they have the virus right now. So, test or no test, we need you to understand you could be spreading it to someone else. Or you could be getting it from someone else. Stay at home,” he said.
As ARC’s previous post describes, amyloidosis patients are an especially vulnerable population, given older age, heart and/or lung involvement, or being on an active treatment that may suppress the immune system. We urge you to follow the CDC’s guidelines for social distancing, being as cautious as you can be.
How long can the virus live on surfaces?
Recent studies have shown that the Coronavirus, COVID-19, can remain detectable in the air for up to three hours, up to 24 hours on cardboard surfaces and up to two to three days on plastic and stainless steel. Given this data, it is of vital importance that individuals and families practice frequent cleaning and sanitizing techniques throughout their home. If you or a family member has gone out into public areas for groceries, gas, or other errands, keep in mind that the virus can be carried into your home. It is best to frequently wash door handles, light switches, faucet knobs, and other high-touch areas in your home, in the event the virus was unknowingly brought inside, even if no one around you has shown symptoms.
How can I grocery shop safely?
A Family and Consumer Sciences Agent out of Utah, Teresa Hunsaker, responded to some commonly asked questions in a recent interview with the Salt Lake Tribune.
It’s possible whoever stocked the store’s shelves was carrying the virus. Maybe another customer picked up your item earlier but decided not to buy it is sick and doesn’t know it yet. “From the carts, to the bags, to the produce,” she said, “everything has the potential to spread the virus.” Below are some recommendations on how to stay safe if you must go to the store:
- Clean shopping cart handles (most stores provide wipes)
- Use hand sanitizer if available
- Keep your distance from other shoppers, and even the cashier if you can
- Use credit cards, not cash
- Wash your produce. If you are at risk or have a compromised immune system, consider frozen or prepackaged produce
- Wipe containers with a soapy cloth or disinfectant wipe before you put the items away
- Sanitize surfaces – countertops, doorknobs, railings, light switches, phone, purse, etc. – anything you’ve touched since leaving the grocery store
Many stores are out of sanitizing products, but soap and water are just as effective. You could also consider adding 1/3 cup of bleach per gallon of water to increase your sanitation efforts. One cup of vinegar per gallon of water would also do the trick.
Is the coronavirus treatable?
An array of drugs approved for other diseases, as well as several investigational drugs, are being studied in several hundred clinical trials that are underway across the globe.
While doctors in China, the United States and other countries have used the drug chloroquine experimentally in COVID-19 patients, there is not yet enough clinical evidence that it’s effective in humans or the management of the coronavirus. Chloroquine and hydroxychloroquine are prescription drugs that have been used to treat malaria, rheumatoid arthritis, and other conditions. In the limited data available, chloroquine treatment for COVID-19 patients demonstrated clinical and virologic benefit, and is now recommended for treatment in several countries, but is not approved for coronavirus treatment in the US, though still possible to receive through various channels if a doctor recommends it.
Remdesivir is an investigational drug with antiviral benefit that is also being studied for its potential therapeutic benefit against the coronavirus. Again, there is limited data regarding its success so far, but can provide peace of mind to the community that treatments may be on the horizon. The FDA released a statement late last week regarding their involvement and goals in product development for COVID-19. “The FDA wants to assure the American public that the agency continues to work with partners across the U.S. government and regulated industry to expedite the development and availability of critical medical products to prevent and treat this novel virus, including repurposing existing therapies that may help treat patients with COVID-19.” To read the full FDA statement, click here.
The FDA also announced on Tuesday that it is expediting the use of blood plasma treatment for patients with life-threatening coronavirus infections. Also known as convalescent plasma, the treatment would involve providing a critically ill patient with the blood plasma of a person who has recovered from the virus.
The Amyloidosis Research Consortium remains committed to providing quality information and resources to the amyloidosis community during this unprecedented time. Please continue to check this post for regular updates, and feel free to reach out to our team should you have any questions. Be well.
March 17, 2020
With rising concerns about the novel coronavirus, COVID-19, the Amyloidosis Research Consortium is providing the following information and resources detailing preventative measures and recommendations for the amyloidosis community. The coronavirus is a highly contagious virus that affects the respiratory system with symptoms such as coughing, runny nose, shortness of breath, and fever. Coronavirus is more likely to affect older adults more seriously, as well as people who have a serious chronic medical condition, like heart disease or lung disease. Many amyloidosis patients fall within these categories, so it’s only natural for patients to be concerned about their risk in a time like this. Additionally, individuals may be at higher risk if they have recently had a stem cell transplant or are on a treatment that is affecting their immune system.
What general precautions should I take?
As you may already know, the virus has demonstrated a wide range of severity; some infected individuals may not report any symptoms at all, while others may get very sick or even succumb to the illness. Given that some individuals with the coronavirus may not experience any symptoms, it’s extremely important to practice caution when interacting with others, for at least the next several weeks.
All individuals should follow the Centers for Disease Control and Prevention (CDC) recommendations include the following:
- Wash your hands often with soap and water for at least 15-20 seconds. If soap and water are not available, use a hand sanitizer with at least 60% alcohol
- Avoid touching your eyes, nose and mouth with unwashed hands
- Take everyday precautions to keep space between yourself and others
- Clean and disinfect frequently touched objects around the home with a standard household cleaner or disinfectant
- Stock up on supplies and medications – enlist the help of a low-risk caregiver or friend to make a trip for you, or consider a delivery service, so you can avoid crowds as much as possible
- If you must leave your home, avoid touching high-touch surfaces such as door handles, elevator buttons, etc. Use a tissue or sleeve to cover your hand if you must touch something
What should I do if someone in my family gets sick?
Choose a room in your home that can be used to separate sick household members from those who are healthy. Identify a separate bathroom for the sick person to use, if possible. Plan to clean these rooms, as needed, when someone is sick.
What should I do if I have medical appointments?
Most centers are postponing non-essential visits and procedures, and these delays should be communicated to you. If you are concerned about an upcoming visit, call your doctor’s office to see if they recommend keeping your scheduled appointment, or if it’s best to hold off.
Telehealth: Many centers are starting to hold telehealth appointments, which allow you to still connect with your physician, but from the comfort and safety of your own home – through the use of video conferencing.
- If you have upcoming appointments, ask your doctor, and check with your insurance, if telehealth visits are an option for you
- Make sure you are enrolled in your providers Electronic Medical Record System, you may be required to do this to use the telehealth service
What if I am on a clinical trial?
This morning, the FDA released a number of recommendations for industry, investigators, and institutional review boards regarding COVID-19 and it’s potential impact on clinical trials, focusing on participant safety and good clinical practices:
“Sponsors, clinical investigators, and IRBs should consider establishing and implementing policy and procedures, or revise existing policy and procedures, to describe approaches to be used to protect trial participants and manage study conduct during possible disruption of the study as a result of COVID-19 control measures at study sites.”
Clinical trial coordinators are currently evaluating the best way to keep patients safe, and may not want you to keep these visits, as they may pose an increased risk of coronavirus exposure to yourself or others. We recommend that you contact your trial coordinator about any upcoming visits, as their preferences and course of action could change as the outbreak worsens.
To read the full FDA recommendations, click here.
What should I do if I am on chemotherapy or have had a stem cell transplant recently?
Dr. Raymond Comenzo, Director of the John C. David Myeloma and Amyloid Program at Tufts Medical Center, and a member of ARC’s Board of Directors, provided recommendations for those receiving chemotherapy, or within a year of SCT, or on daratumumab.
- Follow the guidelines regarding social distance
- Avoid any group events, even family gatherings
- Stay in close touch with your doctor and report any symptoms, keeping in mind that a fever may not happen so a cough, worsening fatigue, muscle aches and any breathing problems may be the first sign of the flu or of COVID-19. If you develop those symptoms, call your doctor and follow their instructions
What should I do if I feel unwell?
Keep your HCP informed of any new symptoms, even those that aren’t thought to be a classic presentation of coronavirus.
For more information on the coronavirus outbreak, please visit the CDC website here.
Well wishes from your ARC team
- Categories
- Lastest Posts
- Phase 3 Clinical Trial of Birtamimab in AL Amyloidosis Fails to Meet Primary Endpoint
- FDA Approves Amvuttra™ (vutrisiran) for ATTR-CM: More Options for Patients
- FDA Approves Attruby™ (acoramidis) for the Treatment of ATTR-CM: A New Option for Patients
- ARC Launches Clinical Fellowship Program to Advance Amyloidosis Care